Electronegativity is a measure of an atom’s ability to attract the shared pair electrons of a covalent bond towards itself. If atoms bonded together have the same electronegativity, the shared pair of electrons will be equally shared. If the electrons of a bond are more attracted to one of the atoms (because it is more electronegative than the other), the electrons will be unequally shared. If the difference in electronegativity is large enough, the electrons will not be shared at all; the more electronegative atom will “take” them resulting in two ions and an ionic bond.
Generally when the electronegativity difference $(\Delta EN)$ is above 1.7, the bond shows ionic character. Similarly, if electronegativity difference $(\Delta EN)$ is below 1.7 the bond show covalent character.

Imagine a game of tug-of-war. If the two teams are of equal strength, the rope stays centered. If one team is stronger, the rope is pulled in that team’s direction. If one team is overwhelmingly stronger, the weaker team is no longer able to hold onto the rope and the entire rope ends up on the side of the stronger team. This is analogous to chemical bonds. If the two atoms of the bond are of equal electronegativity, the electrons are equally shared. If one atom is more electronegative, the electrons of the bond are more attracted to that atom. If one atom is overwhelmingly more electronegative than the other atom, the electrons will not be shared and an ionic bond will result.
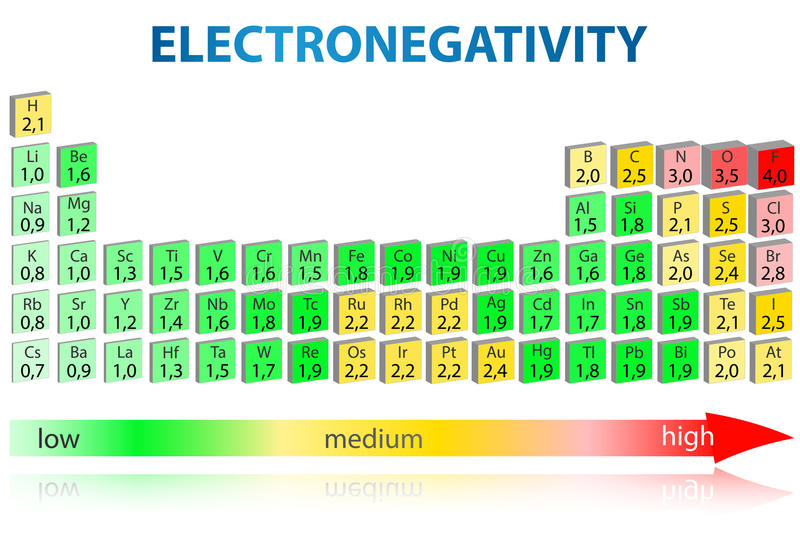
The periodic table below shows the Pauling electronegativity scale. A value of 4.0 is assigned to fluorine, the most electronegative element. As you can see, electronegativities generally increase from left to right across a period and decrease down a group.
Let’s consider an example
Question: Which one is ionic?
A. $Al_2O_3$
B. $AlN$
C. $Al_2Cl_6$
D. $Al_2S_3$
Solution: The easiest way to find out the ionic bond or covalent bond we will check the different in electronegativity of the bonded atoms,
(A). $Al_2O_3$: $\Delta EN=3.5-1.5=2.0$
(B). $AlN$: $\Delta EN=3.0-1.5=1.5$
(C). $Al_2Cl_6$: $\Delta EN=3.0-1.5=1.5$
(D). $Al_2S_3$: $\Delta EN=1.8-1.5=0.3$
So, the $\Delta EN >1.7$ only in case of $Al_2O_3$ i.e. $2.0$. So, in the above options $Al_2O_3$ is ionic.
How to score good in JEE Main?
Finally JEE Main is around the corner. Long wait is now over. Soon all of you will be attempting JEE Main.
Few things you all show remember,
- Avoid silly calculation mistakes
- Avoid silling marking mistakes
- Now its the time only for revision
- If not very much necessary, do not start any new topic or chapter
- Revise what you have completed.
- Give 1 mock test each day and review the solution after the mock test
- Understand your weak points and strong points
- Weak points you have to overcome with every mock test
- Strong points you have to maintain, it will help you in getting good rank
- After giving around 20 mock tests, attempt previous year papers
- If doubts are piling up take the help of mentors.
The most important thing which matters will be the marks scored in JEE Main. It will set the path for your career.
Best of Luck students.
ThinkMerit offers Exam Pack,
- Unlimited doubt support of complex problems by Experts
- 50 Tests
- Mock Test powered by Artificial Intelligence
- Live All India Test
- Previous Year Papers
- Customized test
- Chapter test

Don’t pile up your doubts, clear all your doubts by ThinkMerit Experts.
0 Comments