The Kjeldahl method first came into existence in 1883 and was developed by a Danish chemist named Johan Kjeldahl. This method was specifically developed for determining the nitrogen contents in organic and inorganic substances.
The Kjeldahl method of nitrogen analysis is the Industrial method for calculating the protein content in a variety of materials ranging from human and animal food, fertilizer, wastewater and fossil fuels.
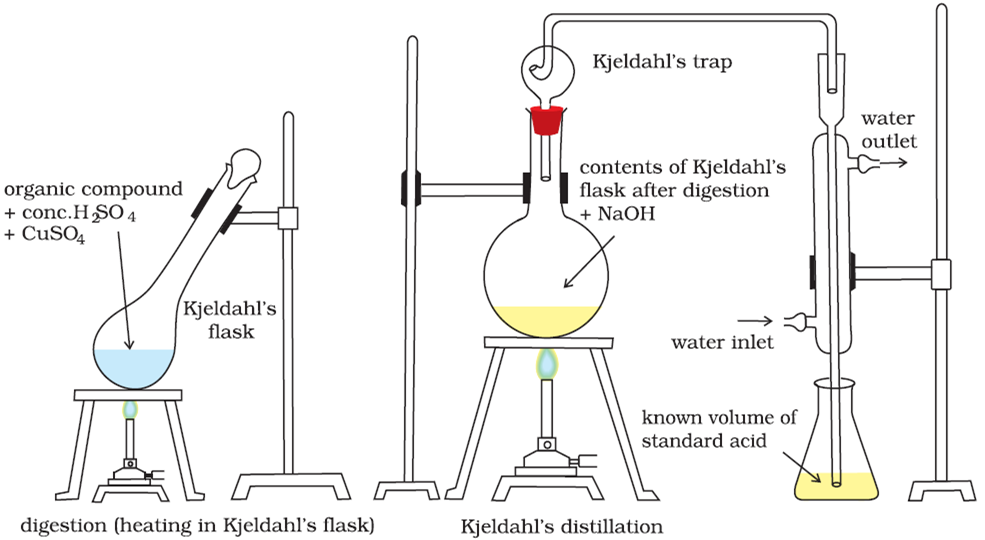
The Kjeldahl method consists of three steps, which have to be carefully carried out in sequence:
1. Digestion: The sample is first digested in strong sulfuric acid $\left( {{H_2}S{O_4}} \right)$ in the presence of a catalyst (like copper, selenium, mercury), which helps in the conversion of the amine nitrogen to ammonium ions in the form of ammonium sulphate.
$${\text{Organic}}\,{\text{compound}} + {H_2}S{O_4} \to [{\text{digest]}}C{u^{2 + }} + {\left( {N{H_4}} \right)_2}S{O_4}$$
2. Distillation: The ammonium ions when treated with sodium hydro-oxide are then converted into ammonia gas, heated and distilled. The ammonia gas is led into a trapping solution where it dissolves and becomes an ammonium ion once again on reaction with $HCl$.
$${\left( {N{H_4}} \right)_2}S{O_4} + 2NaOH \to N{a_2}S{O_4} + 2{H_2}O + 2N{H_3}\;$$
$$N{H_3} + HCl \to N{H_4}Cl$$
3. Titration: Finally the amount of the ammonia that has been trapped is determined by titration with standard solution $\left( {N{a_2}C{O_3}} \right)$ with methyl orange as indicator. It helps in determining the the amount of ammonia with which we can find out the amount of nitrogen.
$$B{\left( {OH} \right)_3} + {H_3}O + N{H_3} \to NH_4^ + + B\left( {OH} \right)_4^ – $$
How to make calculation for Kjeldahl method?
The percentage of nitrogen can be determined as below,
Percentage of nitrogen in the sample $ = \frac{{1.4V \times N}}{W}$
where,
$V=$ Acid used in titration $(ml)$
$N=$ Normality of standard acid
$W=$ Weight of sample $(g)$
Limitations of Kjeldahl method
While the Kjeldahl has become the Industrial method of nitrogen analysis, this method is not suitable for compounds containing nitrogen in azo and nitro groups or in rings (quinoline, pyridine, etc.). It is because in the above cases, the nitrogen cannot be converted to ammonium sulphate.
Exam Pack offer
- Unlimited doubt support of complex problems by Experts
- 50 Tests
- Mock Test powered by Artificial Intelligence
- Live All India Test
- Previous Year Papers
- Customized test
- Chapter test

Don’t pile up your doubts, clear all your doubts by ThinkMerit Experts.
[ninja_form id=1]
0 Comments